 1358
source:
1358
source:
Chengdu, China, July 11, 2024 -- Huitai BioMedicine (HUITAI) announced that the company's first self-developed and globally patented innovative drug, inhaled HTPEP-001 for the treatment of pulmonary fibrosis, completed the dosing of the first healthy subject in Phase I clinical trial at Beijing Chaoyang Hospital. HTPEP-001's mechanism, target, and structure are all first-in-class worldwide.
Back on June 26, the Phase I clinical trial of inhaled HTPEP-001 investigators kick-off meeting was held at Beijing Chaoyang Hospital. This study is a randomized, double-blinded, placebo-controlled Phase I clinical research aimed at evaluating the safety, tolerability, and pharmacokinetics of inhaled HTPEP-001 in healthy subjects. The meeting focused on introducing the background of the project, key technical points of the clinical trial protocol, and ensuring consistency in the implementation of the project operations, with extensive discussions on the clinical operational processes.
This Phase I clinical trial was lead by Professor Tong Zhaohui, Director of the Beijing Institute of Respiratory Diseases and Deputy Director of Beijing Chaoyang Hospital, and Assistant Professor Wang Shumin, Director of the Office of Drug Clinical Trial Institutions. Previously, inhaled HTPEP-001 had obtained the Clinical Trial Approval from the National Medical Products Administration (NMPA) (direct approval) and completed a one-time review from the medical ethics review committee.
Mr Wei Yijun, HUITAI's CEO, stated: "This year, several inhaled drugs have made significant clinical advancements in the treatment of pulmonary diseases globally, and peptide drugs also possess natural advantages in chronic disease treatment. The initiation of clinical trials for HTPEP-001, which combines inhalation delivery, peptide advantages, and a clear anti-fibrotic mechanism, is an exciting milestone. Particularly for patients suffering from pulmonary fibrosis-related diseases, this signifies that they may soon enjoy higher levels of clinical benefits from anti-fibrotic therapy."
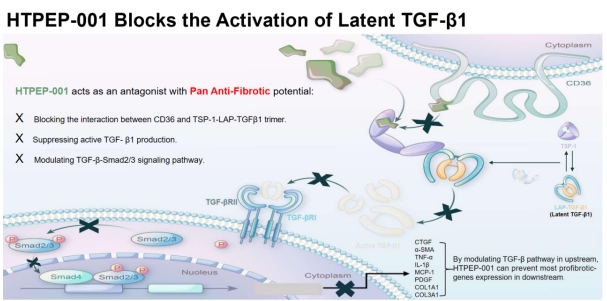
Anti-fibrosis mechanism of HTPEP-001
About Huitai Biomedicine
Chengdu Huitai Biomedicine Co., Ltd. was established in 2017. As an enterprise focusing on the development of anti-fibrosis drugs with core intellectual property rights, HUITAI is dedicated to the research and global commercial development of innovative drugs for fibrotic diseases and solid tumors closely related to fibrosis. HUITAI has established five global first-in-class drug development pipelines dedicated to lung, liver, kidney fibrosis, and solid tumors. Preliminary data has been accepted and presented at international conferences by the American Thoracic Society (ATS) and the American Society of Clinical Oncology (ASCO) twice in a row, receiving high praise from the authors of fibrotic clinical guidelines from both China and the United States. HUITAI is a member of "American Thoracic Society (ATS)" and "Hong Kong Biotechnology Association (HKBIO)", and has been honored as one of the "Top 50 Innovative Biotech Companies in China by KPMG" and one of the "Top 100 Potential Enterprises in the Medicine and Health Industry in Chengdu Hi-tech Zone".





