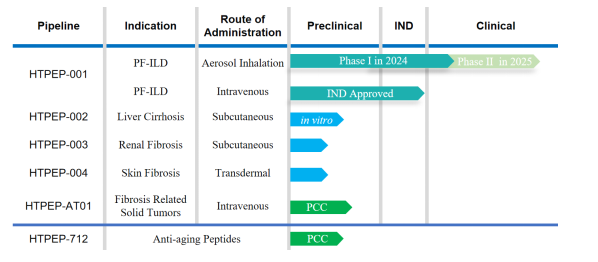
Our flagship asset, HTPEP-001, is an inhibitor of active TGF-β1 that exerts its effects by antagonizing the binding of TSP-1 and CD36. This drug candidate is being developed for administration through two routes: intravenous injection and aerosol inhalation. Pre-clinical data have demonstrated the potent anti-fibrotic effects of HTPEP-001, making it a promising therapeutic option for Idiopathic Pulmonary Fibrosis (IPF).
Early results from the Phase I clinical trial of inhaled HTPEP-001 are encouraging, showing no adverse effects associated with systemic inhibition of the TGF-β signaling pathways. Additionally, the trial has not reported common side effects related to inhalation therapy, such as altered taste sensation or cough.
Building on the success of HTPEP-001, we are expanding our pipeline to include other TGF-β targeted programs aimed at treating conditions like Liver Cirrhosis, Renal Fibrosis, and Skin Fibrosis. We are also exploring a combination therapy, HTPEP-AT01, which combines HTPEP-001 with an anti-PD1 monoclonal antibody (mAb) to address fibrosis-related solid tumors.
Furthermore, we are diversifying our portfolio into the cosmetic medicine field with the development of HTPEP-712, an anti-aging peptide designed to leverage our expertise in fibrosis research. This new venture underscores our commitment to innovation and quality across various healthcare sectors.